The role of the gut and it’s microbiota in neurodegerenative diseases have gain a lot of attention over the years. The hypothesis is that increased gut permeability enables lipopolysaccharide (LPS) and other toxins produced by the gut microbiota to enter the circulation and thence the brain to trigger inflammation and β-amyloid deposition in Alzheimer’s disease. Evidence to support this hypothesis was presented by experts at AAT-ADPD.
Bacteria comprise 98% of the human microbiota, which outnumbers the one trillion host cells by 100 to 1, said Walter J. Lukiw, Professor of Neuroscience, Neurology and Ophthalmology, Louisiana State University, New Orleans, USA; and the ratio of microbiota genes (the microbiome) to host genes is 150 to 1.1 The microbiota is the largest ‘diffuse organ system’ in the human body and the microbiome has been described as our second genome, agreed AnnaMaria Cattaneo, Senior Researcher, King's College London, London, UK.
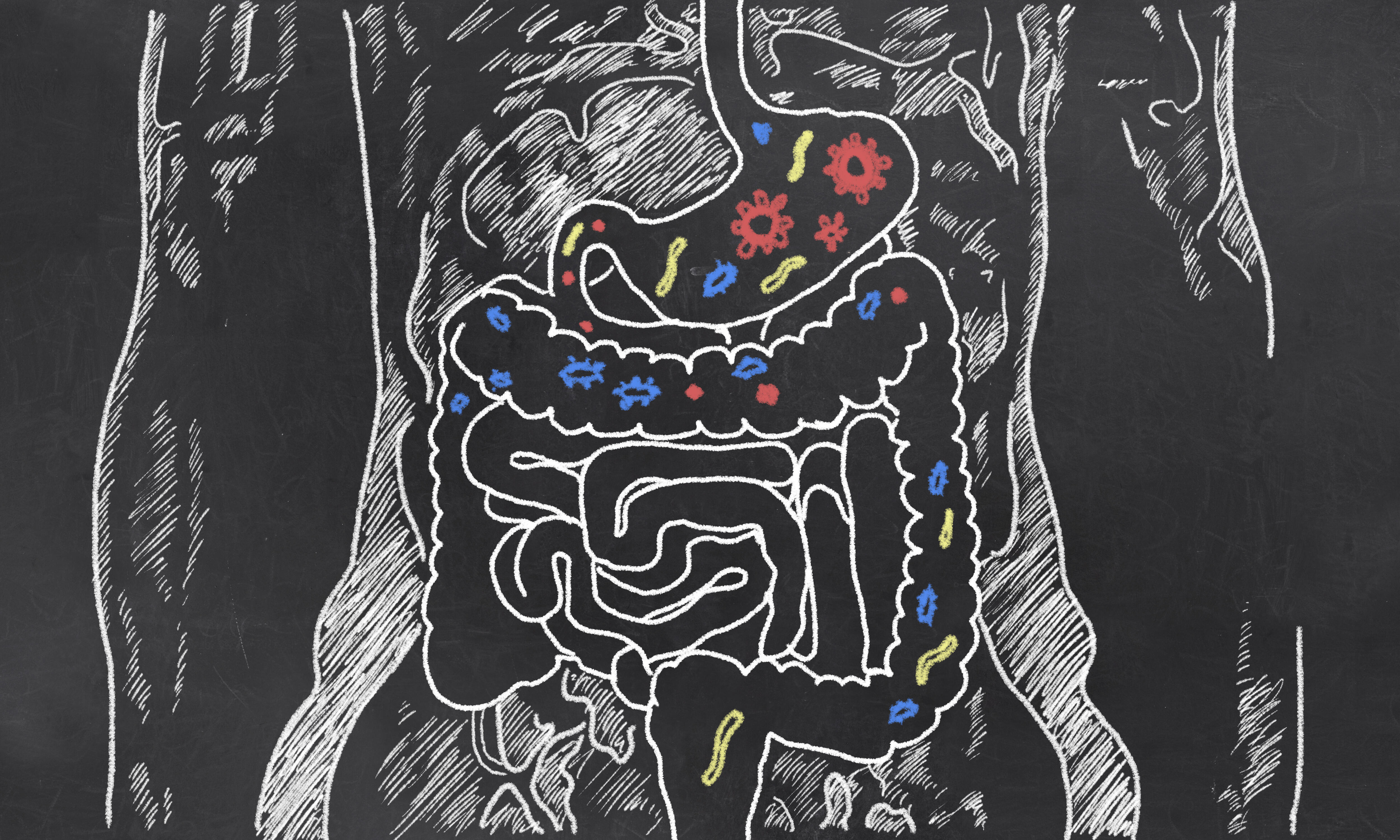
Changes in the GUT MICROBIOTA have been linked to neurodegenerative diseases
An intricate and interlinked symbiotic relationship with many bidirectional pathways exists between the host and the microbiota, which changes throughout life, explained Katia Pane, Research Fellow, University of Naples, Italy. The microbiota-gut-brain axis refers to the bidirectional communication between the gut microbiota and the brain.
Lipopolysaccharide appears to trigger inflammation and β-amyloid deposition
Changes in the gut microbiota have been linked to neurodegenerative diseases. The pathways are not clear, but appear to involve lipopolysaccharide (LPS) in the outer membrane of Gram-negative bacteria and other bacterial proinflammatory toxins.
I Increase in gut permeability could enable LPS to enter the circulation and thence to cross the blood-brain barrier and enter the brain, where β-amyloid could trigger AD pathology, suggested Dr Cattaneo. She noted that blood LPS levels are increased in patients with AD compared with healthy controls.
Aging or disease increases gut permeability so toxins can enter the circulation, and within 15 minutes they are in the brain, agreed Professor Lukiw. Anerobic Gram-negative bacilli in the gut microbiota, such as Bacteroides fragilis and Escherichia coli, secrete the proinflammatory toxins amyloid, endotoxin, LPS, and sncRNA, he added.
Aging or disease increases gut permeability so toxins can enter the circulation
Microbiome-derived LPS is associated with specific downregulation of neurofilament light polypeptide leading to loss of arborisation of neurons in AD, Professor Lukiw explained. Bacterial LPS can be detected in brain lysates from the hippocampus and superior temporal lobe neocortex of AD brains in higher amounts than in age-matched controls.2
Human neuronal-glial cells incubated with LPS exhibit significantly decreased output of DNA transcription products, he added.
Microbial metabolites, protein segregation and neuroinflammation contribute to the neurodegenerative pathway, explained Dr Pane, and potential triggers might be an immune response mediated by LPS or microbial metabolites travelling along the vagus nerve. The term MAPRANOSIS – Microbiota Associated Proteopathy And Neuroinflammation + osis – was proposed in 2017 to describe the process.3
A specific subset of the gut microbiota may promote brain inflammation
The abundance of proinflammatory gut microbiotaEscherichia/Shigella is increased in patients with cognitive impairment and brain amyloidosis when compared with β-amyloid-negative patients and controls, said Dr Cattaneo. In contrast, a decrease in the abundance of the anti-inflammatory Eubacterium rectale is seen in patients with cognitive impairment and brain amyloidosis.
These findings are associated with higher blood levels of the pro-inflammatory cytokines interleukin (IL)-1β, IL-6, chemokine ligand 2 (CXCL2), and NLRP3; and a lower blood level of the anti-inflammatory cytokine IL-10 in patients with cognitive impairment and brain amyloidosis.4
Inflammation may be key in AD pathogenesis
Decreased gut microbiota diversity and a distinct gut microbiota composition has also been reported in patients with AD compared with age- and sex-matched controls, with an increased abundance of Firmicutes and a decreased abundance of Bacteroidetes and Bifidobacterium.5
Manipulation of the gut microbiota may prevent or halt neurodegeneration
A low-fibre, high-fat diet increases the abundance of Bacteroides fragilis
A low-fibre, high-fat diet increases the abundance of Gram-negative B. fragilis, said Professor Lukiw; and Dr Pane suggested that external factors such as diet will inform strategies for the treatment of microbiota-linked disease.